'Hallmarks of Aging' Series Part IV: Stem Cells and Intracellular Communication
The first two of the four "integrative" aging hallmarks.
Greetings!
Last week, I published part III in an ongoing series on the Hallmarks of Aging.1
If you want to read (or re-read) parts I and II, I’ve also provided links to those posts below.
Part I
Part II
This week, we’ll discuss the first 2 of the 4 “integrative” hallmarks: stem cell exhaustion and altered intracellular communication.
Stem cell exhaustion: an overview 🩹
Stem cells are undifferentiated, self-renewing cells with the remarkable ability to give rise to various specialized cell types in the body — they’re the foundational building blocks for tissue development, growth, repair, and maintenance. Stem cells can divide and differentiate into specialized cell types, such as nerve cells, muscle cells, or blood cells, enabling them to replenish damaged or aging tissues and contribute to tissue regeneration.
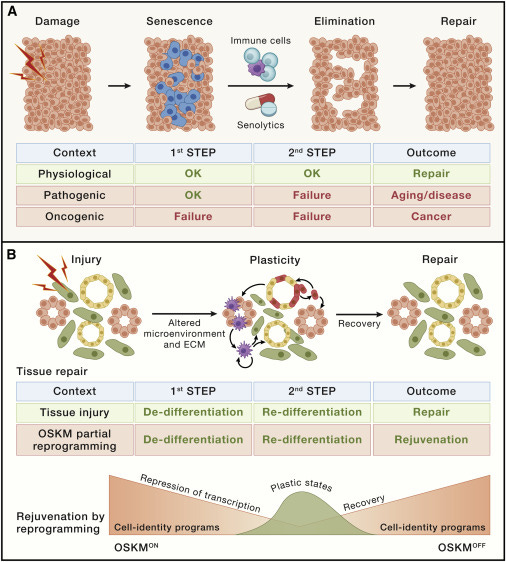
Injury-induced plasticity of stem cells plays a significant role in tissue repair, involving the de-differentiation of non-stem cells and the reactivation of embryonic and stem-cell transcription programs. This reprogramming allows cells to regain their plasticity and contribute to tissue repair.
Aging has noticeable effects on tissue renewal and repair mechanisms.
Notably, the loss of plasticity with aging might have pronounced effects on the overall aging process. Stem and progenitor cells are subject to the same hallmarks of aging as non-stem cells, emphasizing the importance of preserving their function to counteract aging-related decline. The concept of "cellular reprogramming" as a means to address the diminishing function of stem cells during aging is an emerging area of research.
Fixing stem cell exhaustion through reprogramming 🛠️
Cellular reprogramming involves converting mature adult cells into pluripotent stem cells, known as induced pluripotent stem cells (iPSCs), through the introduction of specific transcription factors which are known as the Yamanaka factors (named after their discoverer professor Shinya Yamanaka) — OCT4, SOX2, KLF4, and MYC. This process leads to a reversal of aging features within the cells, characterized by a reduction in markers of aging like p16, an extension of telomeres, and a resetting of the DNA methylation clock.
Partial reprogramming, a transient state of the reprogramming process, has the ability to rejuvenate cells by resetting their epigenetic and transcriptional profiles. This process can be halted at an intermediate stage and allows cells to revert to their original identity while retaining the rejuvenated characteristics.
Transient/partial reprogramming has been shown to enhance tissue repair in aged mice, restoring their repair capacity to levels similar to young individuals. This repair capacity is evident across various tissues, including the pancreas, skeletal muscle, nerve fibers, eye, skin, heart, and liver. Additionally, transient reprogramming can partially reverse age-related tissue dysfunctions, such as visual acuity and loss of neurogenesis in the hippocampus. Some tissue damage conditions, like traumatic brain injury and skin wound healing, also benefit from transient reprogramming.
Altered intracellular communication: an overview 📵
Aging is marked by progressive changes in intercellular communication that disrupt the system's balance and compromise our body’s regulatory mechanisms. This includes deficiencies in neural, neuroendocrine, and hormonal signaling pathways such as adrenergic, dopaminergic, insulin/IGF1-based, and renin-angiotensin systems (which regulate blood pressure), as well as sex hormones linked to reproductive decline. While the underlying causes are often intrinsic to cells, such as the senescence-associated secretory phenotype (SASP), these disruptions accumulate to form a distinct hallmark.
This hallmark serves as a bridge between cell-intrinsic features and meta-cellular hallmarks, contributing to chronic inflammatory reactions, weakened immunosurveillance against pathogens and early cancer cells, and disturbances in the bidirectional communication between the human genome and the microbiome, ultimately leading to dysbiosis.
The central nervous system exerts control over various aspects of aging that impact peripheral organs, a major one being the control and regulation of circadian rhythms.