'Hallmarks of Aging' Series Part V: "Inflammaging" and Gut Dysbiosis
Wrapping up the "integrative" aging hallmarks.
Greetings!
Last week, I published part IV in an ongoing series on the “Hallmarks of Aging.”1
If you want to read (or re-read) previous installments in this series, here are part I, part II, and part III.
This week, we’ll discuss the last 2 of the 4 “integrative” hallmarks: chronic inflammation and dysbiosis.
Chronic inflammation: an overview 🔥
Chronic inflammation, often referred to as "inflammaging," is a hallmark of aging characterized by a persistent and low-grade state of inflammation. This chronic inflammatory state has systemic effects and is associated with various conditions, including arteriosclerosis, neuroinflammation, and osteoarthritis, among others.
As people age, the levels of inflammatory cytokines and biomarkers such as C-reactive protein (CRP) and interleukin-6 (IL-6) tend to increase and are predictive biomarkers for all-cause mortality. Chronic inflammation is closely linked to age-related changes in immune function.
These age-related immune shifts involve the overactivity of pro-inflammatory T-helper cells. Additionally, there is a decrease in the ability of the aging immune system to perform immunosurveillance effectively, leading to difficulties in eliminating virus-infected, cancerous, or senescent cells. This weakened surveillance contributes to the age-related increase in autoimmune diseases and a worsened ability to fend off diseases.
Moreover, the decline in immune function affects the maintenance and repair of biological barriers, which further exacerbates systemic inflammation in a vicious cycle.
Inflammation is intricately linked to almost all of the other aging hallmarks.
Genomic instability contributes to inflammaging by promoting the translocation of nuclear and mitochondrial DNA (mtDNA) into the cytosol, triggering pro-inflammatory DNA sensors, especially when autophagy, the process responsible for clearing damaged cellular components, is compromised (as is known to be true with aging).
Shortened telomeres can lead to cellular senescence. Senescent cells are a significant source of pro-inflammatory cytokines, further exacerbating chronic inflammation. Epigenetic dysregulation can result in the overexpression of pro-inflammatory proteins. For example, mutations in epigenetic modifiers enhance the production of inflammatory molecules like IL-1β and IL-6, promoting cardiovascular aging.
Dysfunctional proteostasis, the body's ability to maintain proper protein folding and degradation, can lead to the accumulation of misfolded and damaged proteins. These proteins can trigger inflammatory responses.
The activation of nutrient-sensing pathways, such as the growth hormone/IGF1/mTOR axis, due to excessive pro-growth signals, can promote inflammation. Senescent cells accumulate with age and release pro-inflammatory molecules as part of the senescence-associated secretory phenotype (SASP). The SASP contributes significantly to inflammaging.
Mitochondrial dysfunction can lead to the generation of reactive oxygen species (ROS). These ROS can activate inflammatory pathways, further promoting chronic inflammation. The decline in immune function and maintenance of biological barriers due to stem cell exhaustion can favor systemic inflammation. It leads to immunosenescence, affecting the body's ability to eliminate infections, cancerous cells, and senescent cells.
Perturbations in intercellular communication, including changes in immune cell function, can disrupt the body's ability to regulate inflammation properly.
Alterations in the bidirectional communication between the human genome and the microbiome can lead to gut dysbiosis, which is an imbalance in the gut microbiota. Dysbiosis can contribute to systemic inflammation. Finally, disruption of circadian rhythms and dysfunction of the intestinal barrier can further exacerbate inflammaging.
Fixing “inflammaging” ⚒️
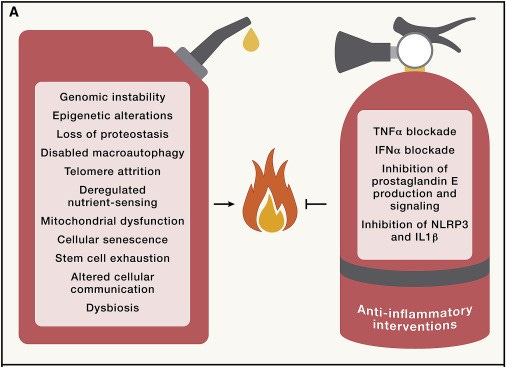
Anti-inflammatory interventions have shown promise in ameliorating age-associated alterations and promoting healthier aging:
For instance, blockade of the pro-inflammatory cytokine TNF-α using inhibitors like etanercept has shown the potential to reverse age-related phenotypes, such as cardiovascular, cognitive, metabolic, and physical aging. Strategies to improve DNA repair mechanisms, such as the senolytic molecule fisetin, have been effective in reducing immunosenescence and age-related damage in various organs, indicating that enhancing DNA repair in immune cells can alleviate aging-related outcomes.
While anti-inflammatory drugs such as aspirin may have beneficial effects on human health, further research is needed to explore their potential value in prophylactic treatments for aging-related conditions, especially in combination with other medications or less toxic anti-inflammatory drugs.
Exercise and “inflammaging” 🏃
Exercise can play a significant role in preventing "inflammaging” through several well-known mechanisms.