Obesity, Diabetes, and the Heart
The intricate web of metabolic and cardiovascular health.
Obesity and diabetes are rampant — more than 40% of adults in the US are obese and approximately 1 in 10 have diabetes. An even greater number have “prediabetes”, indicating a large majority of adults are metabolically unhealthy. These conditions are sometimes considered twin epidemics — as they can (and often do) occur in conjunction. While neither are necessary nor sufficient for the other to occur, the metabolic causes and consequences often overlap.
We usually think of obesity and diabetes in their metabolic terms — using phrases like high blood glucose, insulin resistance, and body fatness to typify them. However, both diseases can have profound adverse effects on the cardiovascular system in addition to their metabolic consequences.
One of the primary findings in both obesity and diabetes is something called cardiomyopathy — a disease of the heart muscle.
Structural changes to the heart in cardiomyopathy
One manifestation of this is what is known as left-ventricular diastolic dysfunction, in which the main pumping chamber of the heart (the left ventricle, or LV) loses its ability to relax. Relaxation (diastole) is necessary for our heart to fill with blood before contraction (systole) ejects the blood out of the aorta and into our circulation.
The obese/diabetic heart has a reduced ability to relax and therefore doesn’t fill properly. In turn, this can lead to systolic dysfunction — a reduced ability to contract and eject blood. The dangerous cycle of diastolic and systolic dysfunction makes it so that circulation in the heart is drastically impaired.
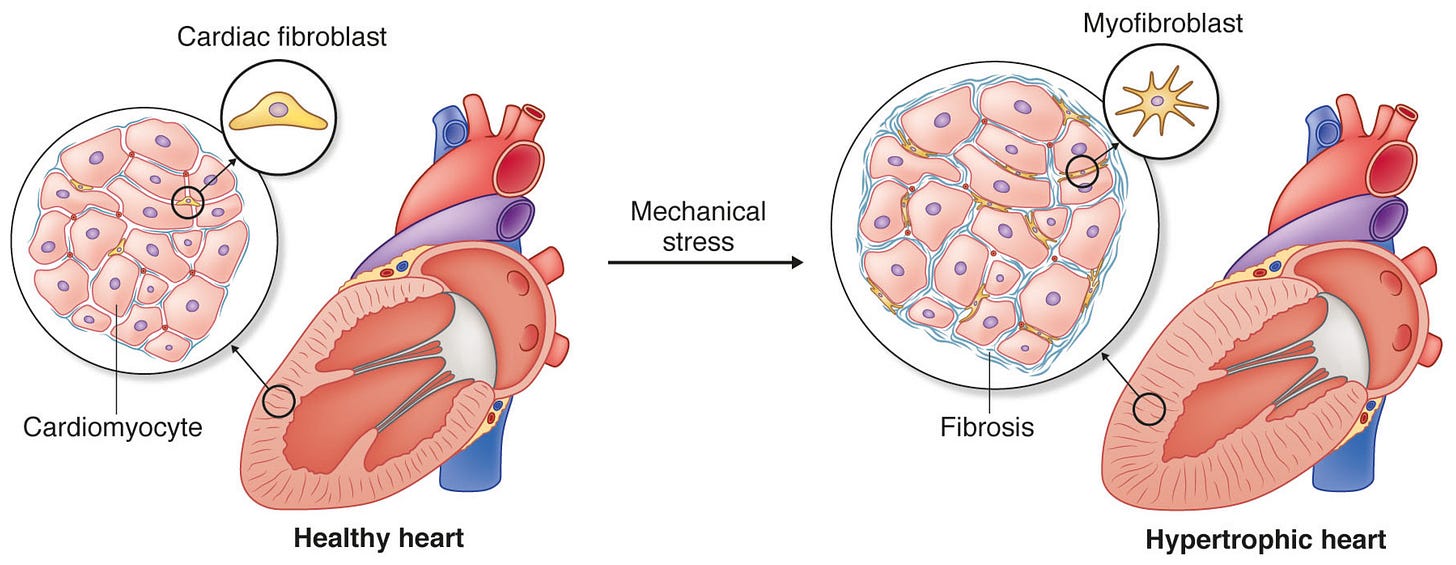
This loss of function is accompanied by significant structural changes to the heart. Diabetes and obesity can cause the walls of the heart to thicken — termed cardiac hypertrophy. These thick, muscular walls lose some of their elastic ability. Stiffer walls are worse at contracting and relaxing. Wall thickening occurs because individual heart cells (known as cardiomyocytes) actually grow larger themselves. As each cell grows (hypertrophies), the wall thickens in proportion.
Ventricle size also increases in diabetic and obesity cardiomyopathy, expanding in size and volume. This process is known as dilatation, and most often occurs in the left ventricle, though the right ventricle also experiences dilatation to some degree.
A final structural change involves a process known as fibrosis. Fibrosis of the heart is implicated in the process of thickening and stiffening. Our heart muscle is surrounded by a tissue layer called the extracellular matrix (ECM) — which is composed of proteins like collagen and elastin, vascular cells, and other immune and fibrotic cells that serve as a structural scaffold for the heart. In diabetes and obesity, the heart ECM becomes fibrotic — an abnormal amount of ECM accumulates and/or the composition changes; leading to a stiffer, fibrotic heart.
This structural remodeling is bad news for the heart. A large ventricle size may prevent adequate contraction, and thicker, stiffer walls prevent adequate contraction and relaxation, which is further impaired by fibrosis. As a result, the heart becomes less efficient at pumping blood, eventually leading to heart failure.1
How do obesity and diabetes lead to cardiomyopathy?
What causes these changes? Some of the pathophysiology is specific to either obesity or diabetes, but a lot of the mechanisms are shared.
In obesity, one of the primary causes of left ventricular hypertrophy is an increased load on the heart. Obese individuals have a higher metabolic activity and increased lean/fat mass, and this increases total blood volume and a hypercirculatory state. An increase in blood volume means that the heart experiences a greater filling volume and elevated systolic and diastolic pressure. The heart must also work harder (i.e. generate more force) to eject blood against these increased pressures. This means that the left ventricle work rate and wall stress are increased, leading to a thicker, hypertrophied muscle. The heart, just like a biceps or triceps muscle, grows larger with “training.”
Increased wall stress and elevated ventricular work rate means the heart has a higher oxygen demand. In diabetes and obesity, as we will see later, oxygen supply may be reduced and therefore, the heart may be “starved” of oxygen.
Metabolic dysfunction
Obesity and diabetes are often characterized by insulin resistance — the muscles and other tissues in the body fail to respond properly to insulin, and therefore blood glucose uptake and utilization are impaired. Insulin resistance leads to hyperglycemia or high blood glucose. High blood glucose (blood sugar) can wreak havoc on the cardiovascular system — it increases oxidative stress and inflammation, reduces blood vessel function, and can lead to the production of advanced-glycation end products (AGEs). AGEs can be considered “sticky” molecules that attach to and cross-link proteins in the body, altering their function.
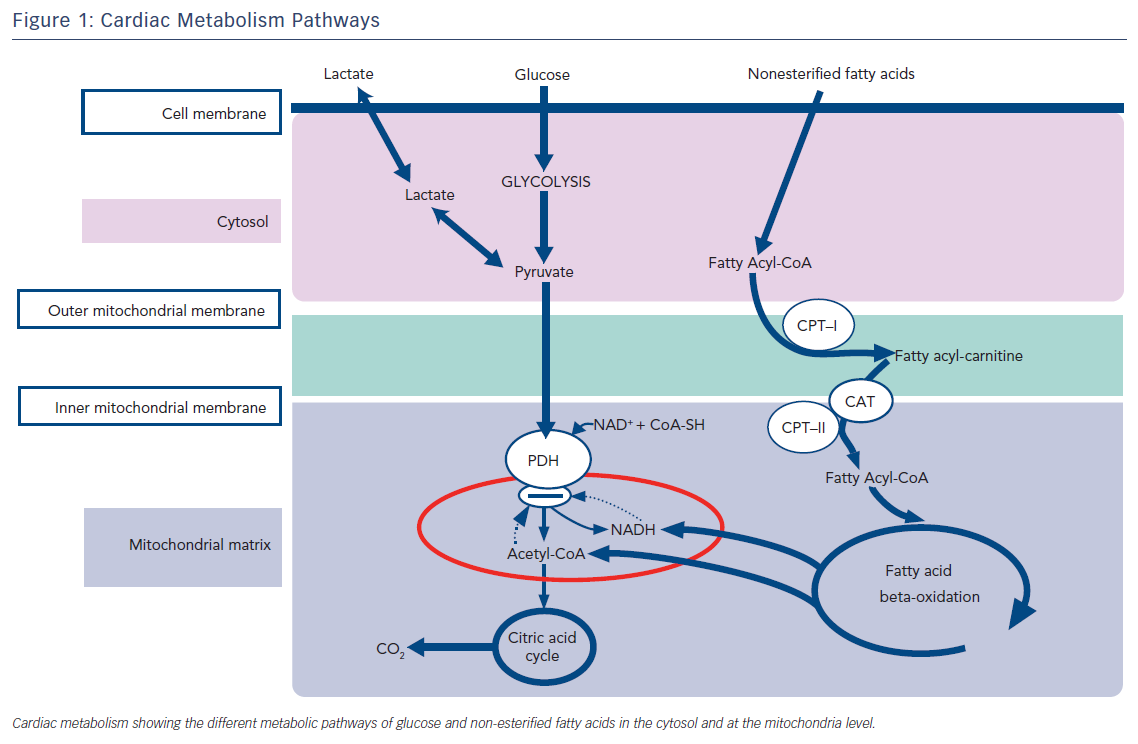
Insulin resistance also leads to a metabolic shift in the heart. Normally, the heart metabolizes glucose (also fatty acids, lactate, and ketone bodies) as a fuel substrate. Without proper insulin action, however, glucose can’t be transported into the myocytes.
Thus, the heart shifts from glucose oxidation to primarily fatty acid oxidation. For one, this increases the oxygen demand of the heart, since fatty acids require more oxygen for their metabolism than glucose. Secondly, an increased flux of fatty acids into the myocytes can lead to something known as lipotoxicity and dyslipidemia; when the supply of these molecules outpaces demand or utilization. Toxic byproducts of fatty acids and triglycerides can promote myocyte cell death and cardiac dysfunction.2
Inflammation and the sympathetic nervous system
Inflammation — characterized by an increased presence of proinflammatory cytokines in the heart, is also elevated in both obesity and diabetes. This can be due to hyperglycemia, insulin resistance, or increased production of inflammatory cytokines from adipose (fat) tissue. Inflammation contributes in part to the cardiac hypertrophy and fibrosis involved in cardiac dysfunction.
Another shared mechanism between obesity and diabetes is the overactivation of neurohumoral systems; including the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system (SNS).
In the body, the RAAS has several effects — causing vasoconstriction of blood vessels and sodium/fluid retention to maintain blood pressure. The main actor here is angiotensin II (ANG II), which causes these effects by binding to its receptors which are present throughout the cardiovascular system.
RAAS is overactive in obesity and diabetes. Several components of RAAS are found in high quantities in adipose tissue, meaning obese individuals have higher levels of angiotensin II than normal-weight individuals (even those with hypertension or cardiomyopathy). In diabetes, RAAS is also overactivated due to hyperglycemia, insulin resistance, and other factors.
When RAAS is chronically overactive, it has adverse effects including vasoconstriction of blood vessels, stimulation of reactive oxygen species (ROS), cell proliferation, and fibrosis. Angiotensin II is a pro-growth factor in the cardiovascular system, and high levels can promote cardiac hypertrophy.
Increased RAAS and insulin resistance can also lead to SNS activation. In the cardiovascular system, SNS activation induces many of the same pathways as does RAAS, including vasoconstriction, inflammation and oxidative stress, and cell proliferation.
Endothelial dysfunction
One final common pathway involved in cardiomyopathy in obesity and diabetes involves endothelial dysfunction. Normally, our blood vessels regulate blood flow and pressure through vasoconstriction and vasodilation (relaxation). A healthy balance of molecules that promote dilation (nitric oxide, NO) and constriction (endothelin-1, ET-1) fine-tunes vascular tone to meet our metabolic needs. Many of the above mechanisms (RAAS, inflammation, hyperglycemia, SNS activation) can impair endothelial function. While it contributes to cardiomyopathy, endothelial dysfunction is also implicated in the progression of atherosclerosis and has other effects such as reducing exercise capacity and cognitive function.
While these are only some of the mechanisms behind cardiomyopathy resulting from obesity and diabetes, the description illustrates a complex interaction between metabolic diseases and the cardiovascular system.
Energy metabolism in the healthy and diabetic/obese heart
Let’s now switch from discussing structural abnormalities in the diabetic/obese heart to some functional changes that occur, in particular those related to energy metabolism.